Source: Tom Williams / Getty
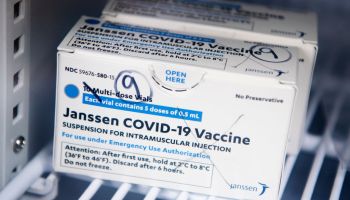
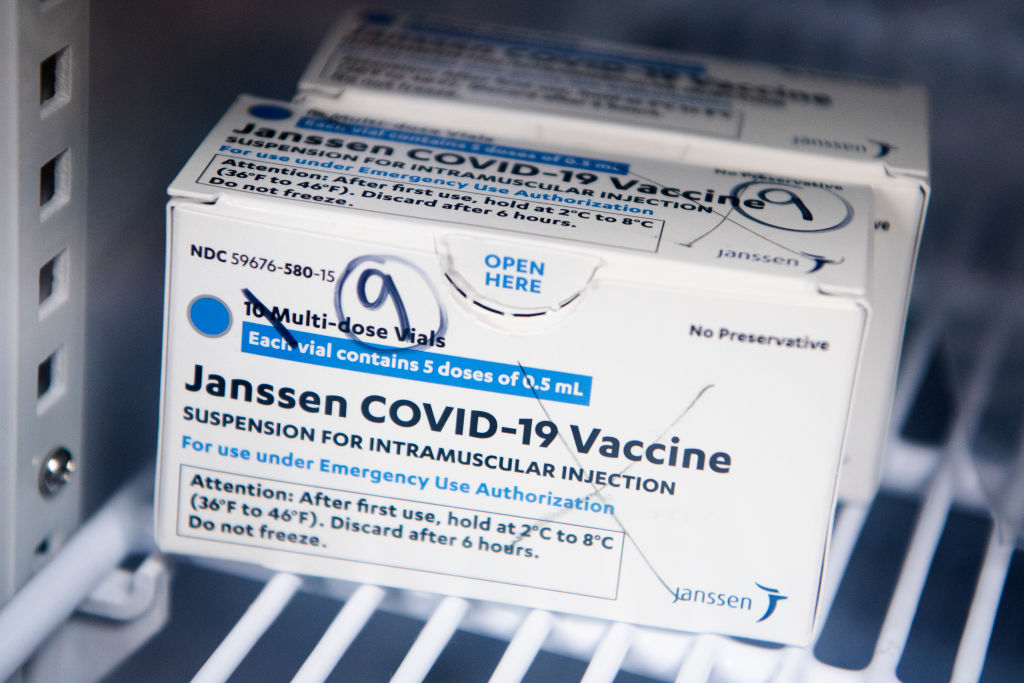
The FDA has announced that the distribution of the Johnson & Johnson COVID-19 will be paused after six women in the U.S. had rare blood-clotting after receiving the vaccine. The women were ranging in ages from 18 to 48, and while six people may not seem like a lot precautions are being taken swiftly. There have been approximately 6.8 million shots given with the majority of people not having issues.
The pausing of the Johnson & Johnson vaccine does not affect the other two vaccines from Pfizer and Moderna. But if you did receive the Johnson & Johnson shot and are having to severe stomach pain, leg cramps, or severe headaches you should call your healthcare provider right away. If you do not know what shot you received, check your CDC vaccination card where it is noted what brand shot you received. If you have an appointment scheduled to receive the Johnson & Johnson COVID-19 vaccine, it is advised to call your provider for further directions.
Get Breaking News & Exclusive Contest in Your Inbox:
The Latest:
- Honda of Cleveland Heights Has Your New Car!
- Black People Experienced Botched Lethal Injections At Higher Rates, New Study Finds
- Quality Life Dedicates Building On Behalf Of Jerry Wade
- Dulcé Sloan Blesses Marjorie Taylor Greene With The Perfect Nickname, “Capitol Hill Karen”
- OHFA Homebuyer Programs
- Creator’s Corner: Brian Courtney Wilson Unpacks What It Means To Elevate
- Ohio Housing Finance Agency- Third Federal Home
- Black Women’s History Month: Korto Momolu Gives Us The Rundown On Her Runway Masterpieces
- Use this Sneaky Hack to Consume Less Sugar
- Solo Dolo No More: Kid Cudi Engaged To Designer Lola Abecassis Sartore
- New Inspirational Music: Koryn Hawthorne, Pastor Mike Jr. & More
FDA is Recommending Pausing the Use of Johnson and Johnson Vaccine in the U.S. was originally published on mycolumbusmagic.com